Oocyte competence prediction
The Aurora Test is a non-invasive gene expression test, performed in the cumulus cells of the Cumulus Oocyte Complex (COC), that provides a ranking for the oocytes of a patient.
The Aurora Test measures the potential of an oocyte to develop into a normal healthy child for patients scheduled for ICSI. After analysing the cumulus cells of each oocyte, the embryo from the oocyte with the highest potential will be transferred into the uterus.
Applying this technology in IVF practice has shown that pregnancy rates have doubled (from 29% to 61%) and live birth increased from 27% to 50% after the transfer of a single embryo on day 3.
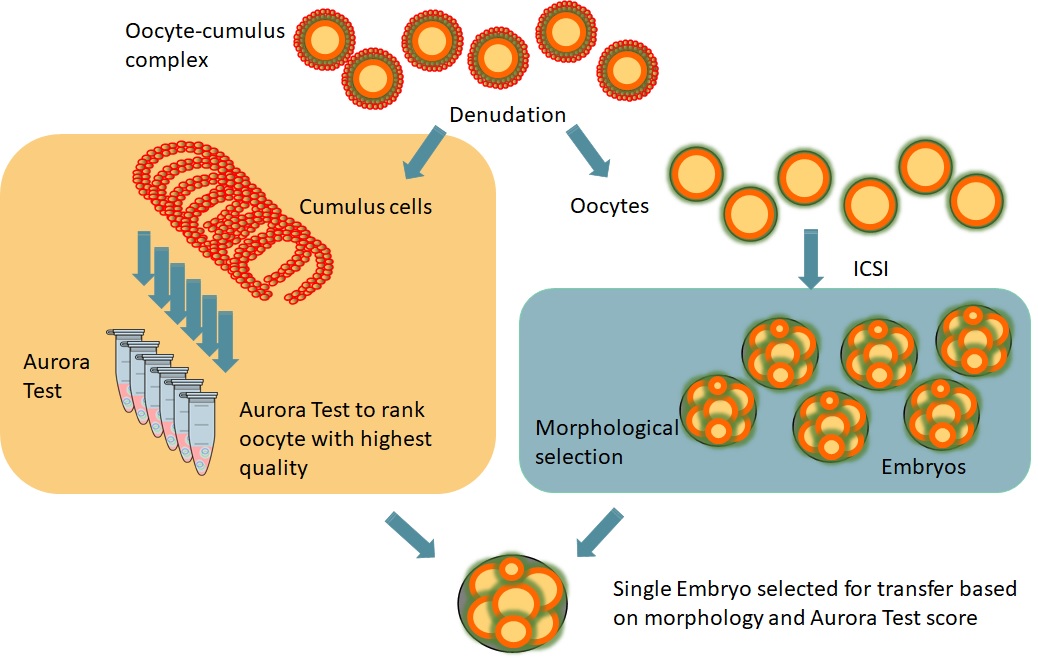
Aurora Test Principle for IVF (ICSI)
To be able to analyse the cumulus cells of each individual oocyte, the classic group oocyte denudation needs to be adapted to an individual denudation procedure.
The Aurora Test is performed on cumulus cells surrounding the oocytes of a patient and it is based on the measurement of the expression of five specific genes.
Cumulus cells are isolated from each oocyte and RNA is extracted from the cumulus cells. cDNA synthesis and real-time PCR are performed using three predictive genes and two control genes. PCR results lead to a quantitative ranking for all oocytes.
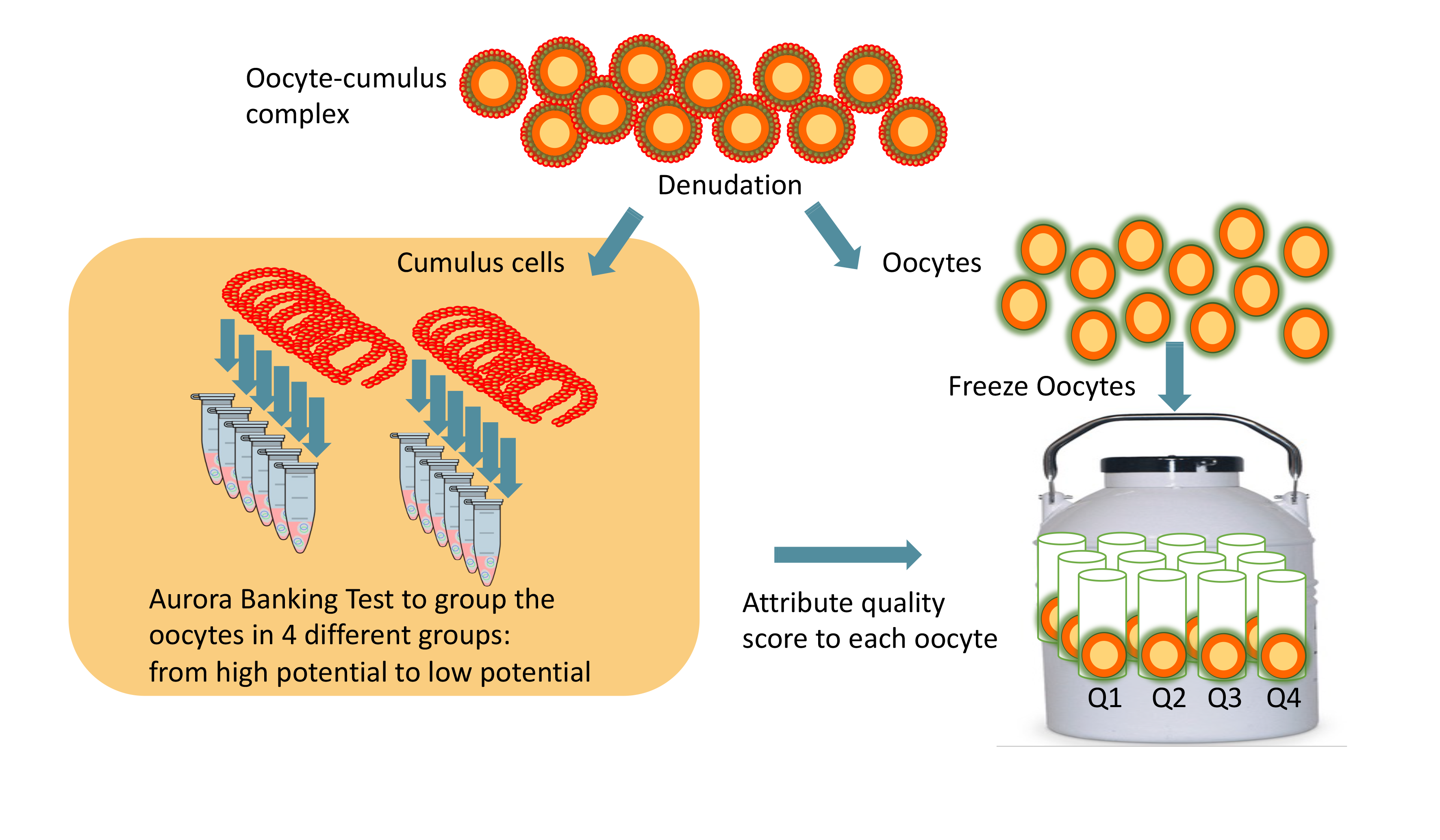
Aurora Test principle for Social Oocyte Freezing
The Aurora Test is performed on cumulus cells from all oocytes from a patient and follows the same molecular testing as for an ICSI patient. The oocytes are ranked, and then, in the future, the highest-ranked oocytes can be used first.
Results clinical trial
Doubled clinical pregnancy rate for Day 3 fresh SET
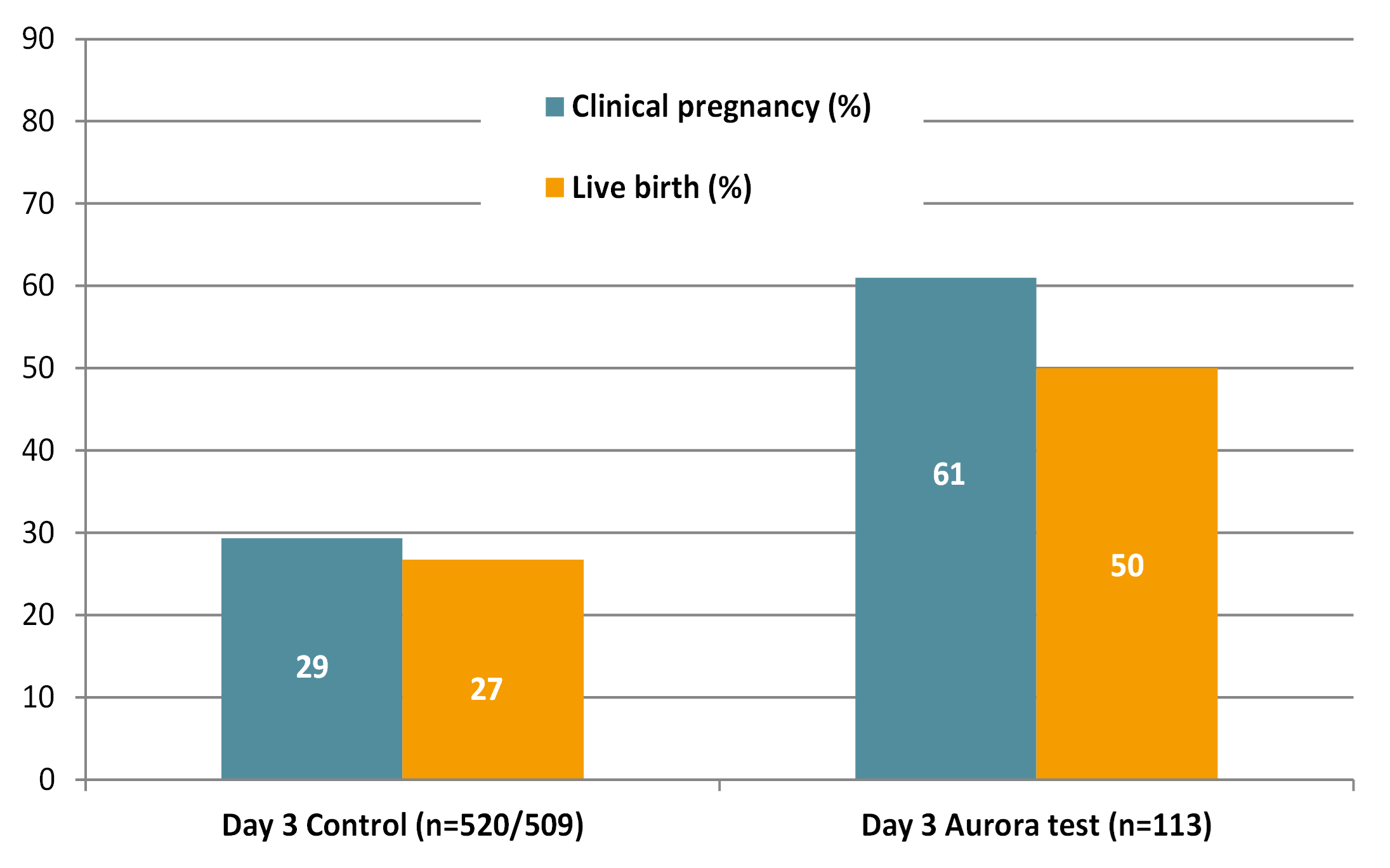
Results of our latest prospective clinical study in Europe showed that in the Aurora Test arm with single embryo transfer (SET) on day 3, the clinical pregnancy rate increased from 29% to 61% in the first fresh transfer cycle (ref.1). These were ICSI patients stimulated with HP-hMG. In the control arm patients also had day 3 single embryo transfer, but with morphological scoring only.
Increased cumulative pregnancy rate
In an earlier study (ref.2) cumulative rates were followed up and the Aurora Test also significantly increased the cumulative pregnancy rate from 56% to 78% in comparison to the day 3 control group when the patient undergoes consecutive cycles.
Increased the live birth rate
The live birth rate increased from 27% to 50% in comparison to the day 3 control group.
Inclusion criteria
The application of Aurora Test is validated for patients with the following criteria:
- Stimulation with HP-hMG hormones (e.g. Menopur) followed by ICSI
- Patient age between 22 to 39 years
- Good ovarian reserve
- Excluding severe male infertility
- Single embryo transfers on day 3 (fresh or frozen) to avoid twin birth
Aurora Test service offered
- DAY 0: Cumulus-oocyte-complex pick-up. Cumulus cells must be removed for each oocyte by an embryologist and individually snap-frozen at -80°C in bar-coded cryovials.
- DAY 0-1: Samples will be transported in a container (dry shipper with N2 or dry ice) to a clinical testing lab nearby which runs the Aurora Test
- DAY 1-2: Aurora Test (RNA isolation, QPCR, and analysis) is done in the clinical testing lab.
- DAY 2-3: Clinical testing lab will communicate Clinical Report to the IVF clinic. The report gives a ranking of all oocytes and specifies which oocyte has the best score.
- DAY 3: The embryologist uses this score along with a morphological evaluation to select the best embryo for transfer.
- In case there is no pregnancy from the first transfer, the supernumerary embryos which were vitrified will also be transferred following the score.
In the future, the Aurora Test application will be broadened to other gonadotrophins.
Literature
- Van Vaerenbergh I, Adriaenssens T, Coucke W, Van Landuyt L, Verheyen G, De Brucker M, Camus M, Platteau P, De Vos M, Van Hecke E, Rosenthal A, Smitz J. Improved clinical outcomes after non-invasive oocyte selection and Day 3 eSET in ICSI patients. Reprod Biol Endocrinol. 2021 Feb 19;19(1):26. https://pubmed.ncbi.nlm.nih.gov/33608027/
- Adriaenssens T, Van Vaerenbergh I, Coucke W, Segers I, Verheyen G, Anckaert E, De Vos M, Smitz J. Cumulus-corona gene expression analysis combined with morphological embryo scoring in single embryo transfer cycles increases live birth after fresh transfer and decreases time to pregnancy. J Assist Reprod Genet. 2019 Mar;36(3):433-443. https://pubmed.ncbi.nlm.nih.gov/30627993/
- Wathlet S, Adriaenssens T, Segers I, Verheyen G, Van Landuyt L, Coucke W, Devroey P, Smitz J. Pregnancy prediction in single embryo transfer cycles after ICSI using QPCR: validation in oocytes from the same cohort. PLoS One. 2013;8(4)
- Wathlet S, Adriaenssens T, Segers I, Verheyen G, Janssens R, Coucke W, Devroey P, Smitz J. New candidate genes to predict pregnancy outcome in single embryo transfer cycles when using cumulus cell gene expression. Fertil Steril. 2012 Aug;98(2)
- Wathlet S, Adriaenssens T, Segers I, Verheyen G, Van de Velde H, Coucke W, Ron El R, Devroey P, Smitz J. Cumulus cell gene expression predicts better cleavage-stage embryo or blastocyst development and pregnancy for ICSI patients. Hum Reprod. 2011 May;26(5)
- Adriaenssens T, Segers I, Wathlet S, Smitz J. The cumulus cell gene expression profile of oocytes with different nuclear maturity and potential for blastocyst formation. J Assist Reprod Genet. 2011 Jan;28(1):31-40
- Adriaenssens T, Wathlet S, Segers I, Verheyen G, De Vos A, Van der Elst J, Coucke W, Devroey P, Smitz J. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010 May;25(5)